ANH Core Team
18th December 2008
Europe’s supreme authority on food safety— and fluoride: Bad science, twisted rules or corporate manipulation?
Summary
Fluoride—the stuff that the dental profession, toothpaste manufacturers and some governments have been persuading us to use for years in an effort to reduce tooth decay—has now come under the scrutiny of Europe's food safety watchdog, the Parma-based European Food Safety Authority (EFSA). EFSA have just released their Scientific Opinion on the safety of a particular form of fluoride, calcium fluoride, when used for "nutritional purposes" in food supplements.
In our review, we assess EFSA's role in general and then, more specifically, its Opinion on calcium fluoride. We argue that the EFSA is working outside of its remit in evaluating calcium fluoride because its intended use is not nutritional, but rather medicinal. Ever since two particularly nasty forms of fluoride, sodium and potassium fluoride, made it on the two EU Directives, one dealing with foods for particular nutritional uses, the other with food supplements, It was obvious something odd was going on. It seems ever more clear now that this system exists to give some of the biggest personal care and pharmaceutical manufacturers a simple, low cost regulatory route to getting their toxic products to the masses, whether we're looking at effervescent tablets, caplets or other delivery systems.
We challenge EFSA's approach to assessing our fluoride intake from drinking water sources, toothpastes and mouthwashes, using the case of the hypothetical Mr Regular—showing how easy it is to exceed safe intake levels even before a supplement taking calcium fluoride is added to the mix.
If you live in an area with fluoridated water, you use fluoridated dental care products, or you are thinking about using fluoride supplements, you should take the time to digest our review. Others of you, who are interested in how the regulators alter their scientific perspectives to suit particular outcomes, will find it just as relevant. EFSA may be able to hide behind the fact that it was Blackmores, the Australian supplement manufacturer, that submitted the original dossier, but who is really set to gain from this Opinion?
It really does look—as you'll discover—as if EFSA has bent its own rules of risk assessment to give this toxic product the green light, in the primary interests of some large corporations.
It's a pity they couldn't just remind us to brush and floss our teeth properly, and reduce or cut out the sugar from our diet. That would surely be a more responsible approach.
EFSA – the European Food Safety Authority

The European Food Safety Authority (EFSA), from the date of its establishment in 2002, was propelled—ahead of any European Member State government—into being the foremost authority on food safety in Europe. It is formally one of Europe’s institutions, joining the heady heights of the European Commission, the European Parliament and even the European Court of Justice, as part of the elite set of centralised authoritative or rule-making bodies that are increasingly replacing the sovereign rights of European countries.
Feeling pity for EFSA
But sometimes, and somewhat ironically considering the pain this authority is causing health-conscious consumers across Europe, we’ve allowed ourselves to feel sorry for the teaming and ever-expanding pool of Euro-scientists in EFSA’s HQ in Parma, northern Italy. Parma—yes, the very place from which that famous thin-sliced ham originates—is a city nestled in the fertile valley of the Po River in the heart of the richest of all Italian provinces, the Emilia-Romagna.

The picturesque centre of Parma, the city that hosts EFSA
Our pangs of pity have tended to stem from our knowledge of the sheer burden of EFSA’s workload, which includes evaluation of thousands of dossiers ranging from such things as different vitamin and mineral forms (which will be banned across the EU on 1 January 2010 if they get anything less than a positive opinion from EFSA) to an array of food additives and contaminants in food. EFSA scientists are now conducting risk assessments on botanicals in food supplements, and are worryingly declaring many herbs that have safety profiles demonstrating safety over thousands of years of usage in traditional medicinal cultures like Ayurveda and Traditional Chinese Medicine, as “toxic, addictive or psychotropic”.
One of EFSA’s biggest challenges in its young history was its receipt in early January 2008 of some 43,000 dossiers containing substantiation evidence for generic health claims (under the terms of Article 13 of the Nutrition & Health Claims Regulation, No 1924/2006) for foods and food supplement ingredients.
When the pity evaporates
However, for many of us, our pity for the apparently over-burdened EFSA evaporated rapidly when EFSA issued negative opinions on valuable forms of vitamins and minerals, such as many of the most commonly used forms of vanadium used by many to help manage blood sugar levels, or on half of the key compounds within natural vitamin E (all four tocotrienols).
The pity even morphed into frustration or downright anger when EFSA’s accomplices at the European Commission decided, back in early 2008, to eliminate over 40,000 of the 43,000 generic health claims applications, many of them that had been painstakingly and sometimes expensively collated, by suddenly moving the goalposts months after the applications had been received. A new essential criterion was suddenly introduced; one that wasn’t made clear before companies spent valuable resources preparing their dossiers prior to the late 2007 deadline given by most Member States. The criterion in question was a new requirement for human studies; any dossier not supported by human studies, irrespective of the amount of supporting evidence from biochemical, animal or other non-human studies, was simply cast out, one hopes into some efficient paper recycling system. This poor procedural and administrative process represents a travesty to the time, resources and paper that has been wasted. Small businesses, not the vast transnationals of Big Food and Big Pharma, were the primary losers, other than the forests that provided the reams of paper. And no one received as much as an apology for the radical shift in the goalposts that hurt, most of all, innovative smaller businesses with limited resources.
EFSA goes transparent on transparency
One positive recent outcome is the European Commission’s decision to issue a public consultation on EFSA’s transparency. EFSA didn’t like it the last time ANH shone a spotlight on their activities— and now they’re going to have the whole world scrutinising them! We know that EFSA claim to work hard to ensure their openness and transparency, but just how transparent will be the evaluation of the public comments on EFSA’s transparency? Let’s hope it’s not a(nother) stitch up!
Fluoride: why isn’t it a drug?
This one really takes the mustard! EFSA have released its opinion on the safety of food supplements containing calcium fluoride, adopted on 27 November 2008. The Opinion is officially titled: "Calcium fluoride as a source of fluoride added for nutritional purposes to food supplements."
“Nutritional uses for fluoride?!!!” I hear you say. Yes. Food supplements containing an ingredient that has little benefit, unless delivered at very low doses in its naturally-occurring forms (like calcium fluoride itself), where it plays a role in bone formation and mineralisation. But fluoride food supplements are not used for this purpose (as that would definitely make them medicines), their intended usage is based instead on the poisonous properties of fluoride salts which they contain—in other words, usage at dosages that kill bacteria in the mouth and thus, allegedly, prevent tooth decay (dental caries). The proposed delivery systems, as given in EFSA’s opinion, are “tablets, caplets, capsules, chewable tablets, effervescent powders and liquids.”
The real problem here is that these calcium fluoride supplements that are meant to be good for our teeth are intended to be used over and above those levels of fluoride that we already ingest in our diets, through drinking water and via dental or oral hygiene products.

Looks clear—but is it clear of fluoride? You could be ingesting more than 1 mg of fluoride for every litre of tap water you drink...
Now, why on earth is Europe’s premier food authority on food safety evaluating the safety, under the terms of the EU Food Supplements Directive, of something that is clearly not a food ingredient, and can be categorised in no other way other than as a medicine!
The definition of a medicine in European law (Directive 2001/83/EC, amended by Directive 2004/27/EC) is any substance that is used either to “treat or prevent disease” or one that is used “with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action” (Article 1(2), Directive 2004/27/EC). If you haven’t got it already, that makes all foods fall under Europe’s drug definition, and it is only exclusions for products that are regarded as “clearly” foods, food supplements, cosmetics or medical devices, that can escape the clutches of this law (Recital 7, Directive 2004/27/EC).
So, banzai! Fluoride cops it on both counts! Its only purpose is to prevent a disease, in this case dental caries, and it definitely has a pharmacological (drug-like) effect that is dose-dependent. Food supplements, under their own definition (Directive 2002/46/EC) should have a nutritional or physiological effect. Well, killing bugs is more biocidal than it is nutritional or physiological, and the EU has a purpose built legal framework for such products, namely the Biocidal Products Directive. But because the bugs otherwise cause a disease, a drug classification has to be closer to the mark.
So why isn’t the particular provision of European drug law that gives these laws supremacy over any other laws in Europe (Article 2(2), Directive 2004/27/EC) tripped into motion?
There are no clear unequivocal explanations anywhere on how this situation has been allowed to continue. But the two most likely candidate hypotheses that fit the facts are:
- the companies who want to fluoridate us through effervescent tablets, caplets, toothpastes, chewing gum and other delivery systems, know very well that their chemical of choice would outright fail any application for a drug licence, which requires extensive studies and clinical trials verifying both its effectiveness and safety, or;
- these same companies don’t want to face the cost burden of paying for a drug licence, and they probably wouldn’t like the public to know that this material they’ve been profiteering from for years is a particularly nasty and insidious poison—a drug even. You see, that might negatively impact sales and force more of us health-conscious consumers into our health stores to find some fluoride-free toothpaste.
EFSA myopia
When you read EFSA’s full opinion on calcium fluoride, something strikes you immediately. Where is the opening section, common to their other opinions on nutrients, that assesses typical, natural intake levels for the nutrient in the diet, including estimates of the Acceptable Daily Intake (ADI)? It’s simply not there. The treatment is typical of a technological food additive and the only indication of the intended use is given in the opening line of the Introduction; “Fluoride supplementation has been used for years essentially to prevent dental caries especially if the fluoride concentration from drinking water is low.” This is akin to saying, "If you're not getting enough of our anti-microbial compound through the water supply, you can take some pills that will help you kill the bugs in your teeth." It's really got very little to do with nutrition.
There are hints as to other uses, but, interestingly, these are not declared, despite a not inconsiderable literature on the role of natural fluorides, like calcium fluoride, on bone formation and repair. But given that EFSA’s terms of reference is to look at risk and not benefits (“why?” you may ask, given that all foods have both risks and benefits and shouldn’t we seek to have an understanding of both sides of the equation?), it would have given them a big headache. Especially when there is an even bigger literature on the adverse risks associated with fluoride exposure, ranging from bone fractures, skeletal and dental fluorosis and osteoporosis risk, through to its carcinogenic and mutagenic potential. Funny then, that EFSA—like the International Agency on Research on Cancer (IARC), based in Lyon, France, whose finding probably contributes to the fact that the majority of water supplies in the USA continue to be fluoridated—should categorise calcium fluoride as a non-carcinogen in humans.
Saying this, the picture is confused by an equivalent body of work, generally funded by pro-fluoride interests, that shows fluorides, but only the natural forms like calcium fluoride, can be used to build bone (although of a different biochemical and structural integrity compared with naturally grown bone) and even reduce cancer risk. It’s no wonder the controversy on fluoride never dies, especially when the studies on natural fluorides are used to support those on synthetic fluorides—including forms like fluorosilicic acid that are generally added to drinking water—which have an even worse safety profile.
Some, of more extreme mind, have of course suggested that fluoridation is part of a sophisticated genocide agenda, but here, respecting ANH’s principle of relying on substantiated evidence, we won’t go that far, as solid evidence supporting this view is wanting.
How does EFSA normally do its risk assessments?
The primary method that EFSA is using for its risk assessments on nutrients in food supplements is quite simple. EFSA evaluates all of the relevant evidence both on exposure and safety, determines what the highest level of exposure from foods, drinks and any other relevant sources might be in particular sub-sections of the population. Then it considers if the inclusion of the nutrient in the food supplement would take you above its particular, agreed ‘tolerable upper level’ of exposure. This is deemed as the highest level of exposure considered safe, so, logically, it is assumed that if you exceed this, you might be exposed to a significant risk.
In the case of calcium fluoride, the risk is not just a minor, transient and reversible risk, such as the loose bowel that accompanies very large, undivided oral doses of vitamin C or a niacin flush. Here we’re talking dental fluorosis, the unflattering mottling of the teeth that we see increasingly in areas where the population is mass medicated with related compounds, such as fluorosilicic acid in the drinking water supply and the sodium monofluorophosphate in a host of dental products common to people's bathrooms.
What EFSA doesn’t elude to is that the very process that leads to the discoloration of children’s teeth—caused by the fluoride ion’s role in removing (demineralising) calcium—is almost certainly going on in less visible parts of our body, namely in our bones. The process is known, unsurprisingly as skeletal fluorosis and it leads to increased bone fractures, just like osteoporosis which has similar effects, although different causes.
But when there are so many possible sources of fluoride, many of which are hard to regulate, exposure assessments are critical, especially if exposures are nearing the ‘tolerable upper level’ of intake. This is most definitely the case with fluoride.
The addition of fluoride to the drinking water supply, its addition to some chewing gums, perceived by most as confectionary products with no restriction on level of intake, and its inclusion on the majority of big name toothpastes and mouthwashes, is scientifically and medically irrational, given the huge variations in consumption rate between individuals occurs. Drinking water, mineral waters and chewing gum are not what one would call accurate delivery systems for a drug.
Mr Regular—and his daily fluoride hit
In its calcium fluoride opinion, EFSA looks at intake data from food and drink, mineral waters and tap water, but it doesn’t focus at all on population groups that might be exposed to all sources.

Multiple sources of fluoride in the bathroom
Imagine a day in the life Mr Regular, who has yet to become familiar with the risks associated with lifetime, all-source, cumulative exposures to fluoride. He gets up in the morning and freshens his mouth with a fluoridated toothpaste and mouthwash. He drinks several cups of tea during the day, where he’s ingesting fluoride both from the tap water and the tea, which naturally contains relatively large amounts of fluoride. In fact the World Health Organization reckons tea drinking alone can cause you to start losing minerals from your bone, leading to skeletal fluorosis. Back to Mr Regular, he’s still not done with this fluoride for the day! He drinks a bottle of fluoridated mineral water to help wash down his lunchtime soup, made, guess what, it might be fresh but it’s still made using fluoridated tap water. After work, he goes down the gym and trains hard, requiring 3 litres of water to quench his thirst. Let’s not forget that during the course of Mr Regular’s busy day, he’s also consuming nearly an entire pack of chewing gum, every piece being laced with more fluoride. He then gets home and cooks his pasta and vegetables in fluoridated tap water, sips at a couple of mugs of fluoride-rich green tea as he watches the television news, and, finally, he succumbs to yet more fluoride when he brushes and then flosses his teeth, prior to using a big brand fluoridated mouthwash before bed. Mr Regular, exposed to the subliminal drip-feed from well-funded pro-fluoride interests, has simply not yet been able to appreciate that he had a choice; that he could have—with some effort and at greater cost—sought out fluoride-free toothpaste, floss and mouthwash in awkward corners of the shelves of his out-of-town supermarket and pharmacy complex. Or he could have rummaged around the shelves at his local health store.
When Mr Regular’s tea drinking habit alone put him over the safe limit, it’s not hard to appreciate that he could end up consuming 2, 3 or even 4 times more than the safe limit by the time you’ve considered all the possible sources of intake.
EFSA: twisting its own rules
In brief, one of EFSA’s big decision rules on risk assessment says that if there is any risk that a susceptible sub-population might exceed the scientifically determined upper safety limits—once dietary intakes from all sources have been taken into account—supplementation cannot be supported (Article 5, Directive 2002/46/EC). EFSA don’t want us, in good faith, it seems, to run the risk of consuming that additional amount that tips us over their pre-determined upper safety threshold. And it is exactly this concern that caused EFSA to judge negatively the sense in taking those forms of vanadium that are more bioavailable than those in food sources, such as shellfish.
But why does EFSA—arbitrarily it seems, with no explanation—suddenly change the rules for fluoride? EFSA even goes as far as saying in its opinion that it acknowledges that around 15% of UK children between the ages of 1 and 8, who are already exposed to fluoridated drinking water, could exceed the safe level if they were exposed to calcium fluoride “food supplements”.
The forgotten children: of Ireland and the UK
Wait on—these British children are a recognised sub-population and, what’s more, they are highly susceptible because, as kids, they’re busy laying down bone and other tissues while they’re growing. But EFSA, in contravention of the process it’s used to restrict us from many considerably safer and more beneficial nutrients, has ridden roughshod over these young British kids that it has itself identified. Making matters worse, it has shown a total disregard for Irish children, who are faced with one of the highest rates of dental fluorosis in the world, meaning that they are already well into risk territory. This is because of the Irish government’s policy, unique across Europe, to fluoridate the majority of the country’s water supply. Yet, based on EFSA’s positive opinion, companies will carry on lacing fluoride into a wide range of products and Irish kids will continue to be coaxed by lurid advertising into consuming yet more fluoride in chewing gums and toothpaste.
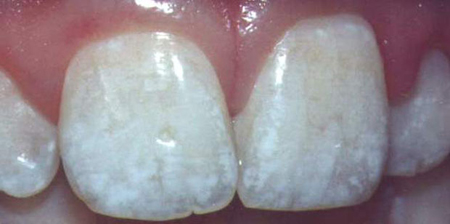
Classic symptoms of dental fluorosis in a child
It seems that there is a multiple agenda. If the scientific method or procedure used by a regulator is constant, we are able to engage in constructive criticism of it, with a view to helping shape a more robust system that ensures consumers are adequately protected, while particular health-giving products are not forced to be withdrawn from the market.
But now we have something different: The banishment of precautionary risk assessment principles—when this provides a more suitable outcome—that we were beginning to think were indelibly embossed into the fabric of EFSA.
The common thread
The only common thread we are able to identity at this point, is that EFSA appears to be doing exactly what other regulators such as the European Commission itself, the UK medicines regulator, the Medicines and Healthcare products Regulatory Agency (MHRA) and the US’s Food and Drug Administration (FDA) have been exposed for doing in the past; working—ultimately—in the interests of the biggest corporations on the planet.
Click here to go back to ANH Homepage








Comments
your voice counts
There are currently no comments on this post.
Your voice counts
We welcome your comments and are very interested in your point of view, but we ask that you keep them relevant to the article, that they be civil and without commercial links. All comments are moderated prior to being published. We reserve the right to edit or not publish comments that we consider abusive or offensive.
There is extra content here from a third party provider. You will be unable to see this content unless you agree to allow Content Cookies. Cookie Preferences