Content Sections
Jerome Burne and Rob Verkerk podcast/vidcast (30 minutes)
“INCLISIRAN: Should we believe the hype behind the latest cholesterol-lowering jab?”
Listen
or watch
By Rob Verkerk PhD, founder, executive and scientific director, ANH-Intl
The worldwide headlines with titles like the Daily Mail’s “Cholesterol-lowering jab that means never having to take statins again and a breakthrough pill that boosts survival are among astonishing new treatments set to transform the lives of British heart patients” likely herald the start of a new, post-statin era of medicine.
Novartis is the conductor of the show. No more daily statin pills that you sometimes forget to take. Just two jabs a year from your doctor, so you can set and forget. Good value for payers, providers and patients. Promises of no side effects, a welcome boon for those who’ve suffered with muscle pain, increased diabetes risk and other side effects of statins. A new mega-blockbuster drug for pharma in the making, or at least for Novartis.
Too good to be true? We think so. There either just aren’t enough data to validate the claims or promises, or what evidence does exist doesn’t so far support some of the biggest claims around effectiveness (against heart disease, not cholesterol-lowering) and safety. These kind of claims might easily be described as pseudoscience.
Pseudoscience and misinformation fuels the hype
There’s some cracking misinformation being issued to help fan the flames of the hype. This kind of hype is likely viewed as necessary if Novartis is to meet its shareholders expectations, set in November 2019, when the Swiss pharma giant acquired The Medicines Company, the company that has done most of the R&D on inclisiran, for an eye-watering US$9.7 billion.
Check out the 5 ongoing trials in Europe, with no results yet available in the public domain, via the EU Clinical Trials Register. You’ll see The Medicines Company, not Novartis, is the sponsor in all of them, and Oxford University, the co-sponsor in one.
Here’s just three titbits that caught our eye:
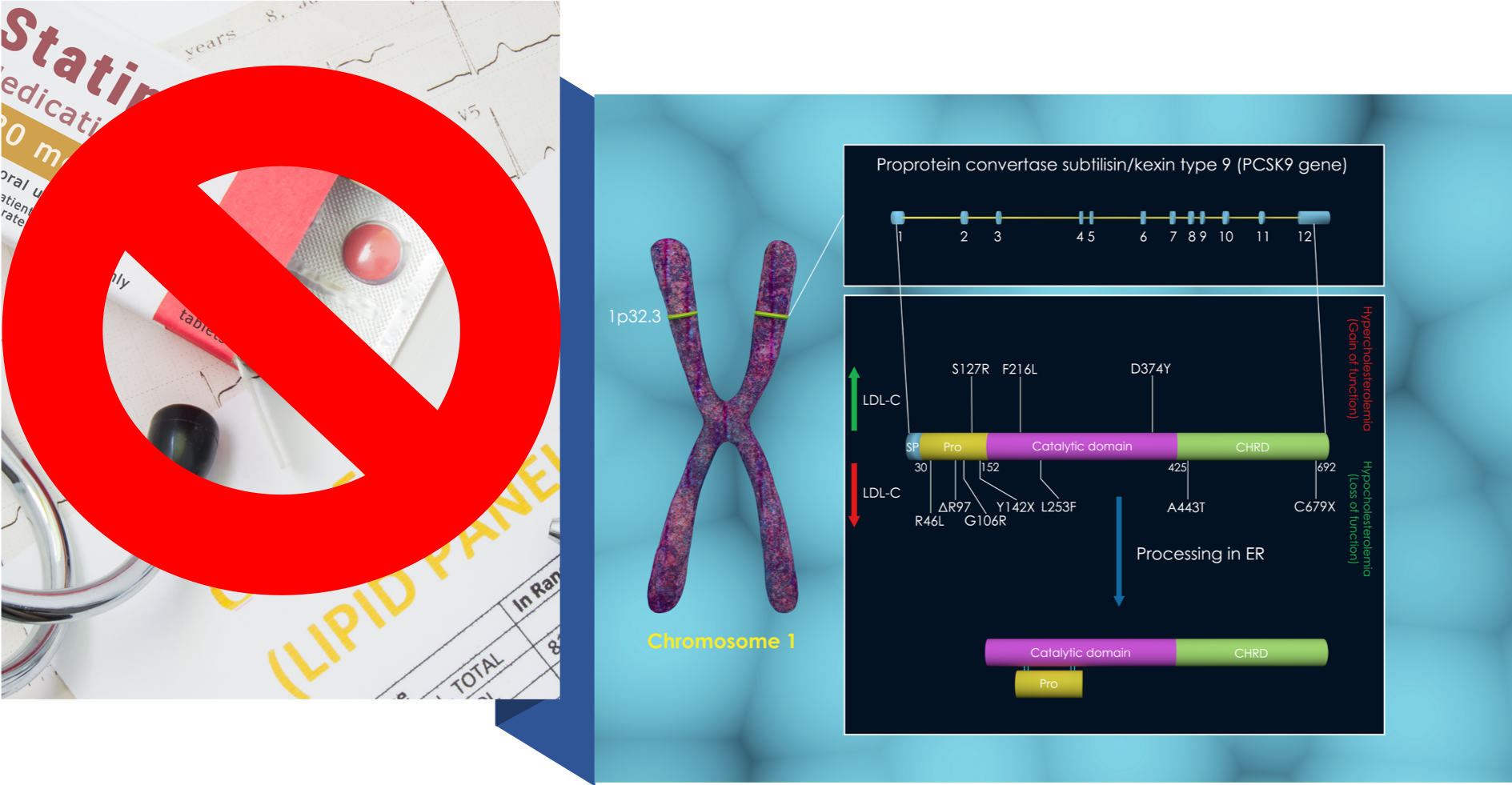
- Professor Ray from Imperial College London who’s leading the UK clinical trials on inclisiran said in the Daily Mail: “…the drug works in a specific way, acting only in the liver and not travelling around the body, there will be even fewer side effects than with statins”. Hold on. This is a new type of gene silencing drug that stops the PCSK9 gene from expressing the PCSK protein. It does this not only in the liver, but also in the brain, kidney and intestinal tissues. Let’s not forget how PCSK9 became such a red hot research area for pharma. It was discovered because mutations in it prevent the liver from removing cholesterol from the blood through the degradation of LDL receptors, leading to familial hypercholesterolemia, interfering with cholesterol and fatty acid metabolism by silencing the PCSK9 gene could lead to issues down the line that won’t be evident without many years if not decades of trials or use. The view that loss of function of PCSK9 doesn’t cause side effects comes from very limited human data that suggests that those who have loss-of-function mutations in PCSK9 are apparently healthy. It totally ignores other research that shows the vital role of PCSK9 in regulating the innate immune response and septic shock outcomes. The idea that the current round of trials that are expected to finish in Europe and the USA by 2023-4 are going to tell us everything we need to know about safety shows us just how little the pharmaceutical industry has learned about drug safety. Or it’s still a case of profit before safety.
- This next one is from Sam Blanchard of the Daily Mail. He says that “The injection inclisiran works in the same way as statins”. It doesn’t. Statin’s are HMG‐CoA reductase inhibitors while inclisiran belongs to a new category of gene silencing drugs, stopping the PCSK9 gene from issuing the enzyme, proprotein convertase subtilisin/kexin type 9 (PCSK9 for short) that is a crucial player in the regulation of cholesterol in the body. Their modes of action couldn’t be more different. Also, PCSK9’s functions go well beyond managing cholesterol homeostatis. Silence something as fundamental as this enzyme and expect complex problems to show up downstream, side effects that would be easy to miss in Phase III clinical trials as has been the case of many drugs before it, such as the 17 drugs, including Vioxx, that were withdrawn from the US market after having been approved as safe by the US Food and Drug Administration (FDA).
- We also get troubled when reporters and even scientists glibly refer to the subcutaneous injection or jab that delivers inclisiran as a vaccine! Inclisiran isn’t a vaccine because it doesn’t contain any substances that incite the immune system to produce antibodies that attack disease-causing organisms or pathogens. The Daily Mail and The Times were two UK papers that did this, presumably believing that the addition of apostrophes around the word ‘vaccine’ somehow absolves them of this cardinal scientific sin. Even my alter mater, Imperial College London, did it back in 2018. It’s particularly disingenuous because work is ongoing on vaccines that use the immune system to disable PCSK9. Progress with developing PCSK9 vaccines aimed at providing long-term cholesterol reduction or reduced heart disease risk has been shown by researchers in the USA, Netherlands and China. You can only wonder what the pro-vaccine corporatocracy might do with this. Could inclisiran shots one day be mandated for all those members of the public found to be in excess of the relatively recently-lowered ‘normal range’ for LDL cholesterol?
Does low cholesterol really mean low heart disease risk?
Another supersized anomaly with the spoon-fed media reporting on Novartis’ inclisiran story is the assumption that lowering cholesterol around 50% will guarantee lowering of heart disease and associated mortality. The drug has already been in the pipeline for around a decade and when it was in its earlier stages of development, the cholesterol hypothesis for heart disease was alive and well. Not so today. As shown in a systematic review published in BMJ Open by an international group of scientists, doctors, including cardiologists, in 2016, there is no clear relationship between circulating LDL cholesterol levels and mortality in older people, the main target group for cholesterol-lowering drugs.
More than this, PCSK9 inhibition doesn’t explain the whole picture with familial (autosomal dominant) hypercholesterolemia. Apart from the much-discussed gain-of-function mutations of PKCS9, there are other mutations, such as mutations in the LDL receptor coding gene or APOB (that encodes apolipoprotein B) that have also been found to be involved.
Profit before health
PCSK9 inhibitors are undeniably a rapidly expanding area of research for pharma. They are widely seen as the next bastion of cholesterol-lowering drugs, coming on line in the nick of time after statins are getting increasingly rejected by a wary public. More and more of us are simply not sufficiently impressed with the evidence or experience of benefit, or we don’t want to continue to have to tolerate the side effects that were long denied as serious by the triad of the medical establishment, the manufacturers and government authorities.
By bringing in a new class of drugs to lower cholesterol, Big Pharma will likely do its best to avoid having to admit to losing the war on statins. Lo and behold, something bright, shiny, bigger and better, replete with long patents, came along and helped divert our focus.
The (unspoken) dietary and nutraceutical option
Heaven forbid there might be ways of managing cholesterol, heart disease and even PCSK9 gene expression that are natural! Surprise, surprise, of course there are; ingredients that are present in our food supply, also available as nutraceuticals, have been shown to be very effective in helping to regulate cholesterol in the body, and prevent it from oxidising unnecessarily.
Also, when one comes at the problem from a systems biology viewpoint, as the cutting edge of the integrative, natural and sustainable medicine community do, cholesterol is not demonised. In fact, it is celebrated for its multiple functions in the body, including through its role as the precursor of all steroid hormones including vitamin D, in the structure and function of all cell membranes and gates in membranes, and for cell signalling. Put simply, cholesterol is essential to all animal life. Labelling LDL cholesterol as ‘bad’ cholesterol, as Novartis has done in all its press releases, is a misnomer. More pseudoscience.
Examples of food ingredients or nutraceuticals that have been shown to help maintain healthy cholesterol metabolism include:
- Berberine
- Prebiotics and probiotics
- Plant sterols (phytosterols)
- Vitamin C
- Green tea (epigallocatchin gallate [EGCG])
- Red yeast rice, which contains a fungus (Monascus purpureus) that provides monacolin k, that can be considered a ‘natural statin’.
When cholesterol isn’t seen as the ‘bad guy’, more effort can be expended to protect it, especially avoiding the unnecessary and preventable oxidation of LDL to form so-called oxidised LDL (ox-LDL). When very small LDL particles are oxidised (ox-VLDL), this can become an especially important risk factor for metabolic syndrome and the development of foam cells in arteries. The formation of these foam cells is a precursor to atherosclerosis, the process that gives rise to coronary heart disease, the leading killer and form of heart disease in most of the Western world.
By way of a few examples, coming to the rescue as antioxidants that can reduce or prevent the formation of ox-LDL and ox-VLDL are:
- Berberine
- Licorice
- Alpha lipoic acid
- Full spectrum vitamin E (rich in gamma-tocopherol)
- Hydroxytyrosol-rich olive oil
- Resveratrol
The list goes on, and, of course, should include all phenol-rich (i.e. coloured) sources of fruits and vegetables or nutrients derived from them.
Clinical trials: you may be at risk
If you’re approached to take part and be paid for being part of a clinical trial for inclisiran, you can perhaps now make a more informed choice.
Please share this article widely as you won’t find this information in the newspapers, or on the major television or radio channels.
For more information about Novartis and its aspiration for inclisiran as the next mega-blockbuster drug
Explore the podcast, presentation slides and Q&A from Novartis’ investors meeting in November 2019, following the acquisition of The Medicines Company.
Read more on award winning medical and health journalist, Jerome Burne's blog - Health Insights UK
Zoë Harcombe PhD takes a deep dive behind the headlines







Comments
your voice counts
06 February 2020 at 10:36 am
Thank you for making us aware of this new way of treating high cholestrol. I do not take statins but some members of my family do. What happens if the cholestrol levels come down too low or the drug does not suit you have they got an antidote.
08 February 2020 at 7:52 pm
It's amazing to see how 'science' proceeds on the basis of faulty premises - especially when funded under cover of 'defeating the bad guys' - in this case flagged to cholesterol.
No matter how respectable or well intentioned, framing in a false model will distort not only results, but the ongoing interpretation of results - until the insanity grows to demand truth be a coercive narrative dictate of a top down 'consensus' clinging to the white coat as a vestige of 'authority'.
Having instilled and conditioned fear in the shoal to the point of undermining residual immunities - I foresee a 'feeding frenzy'. The phrase keep your head, means keep it connected to reality - which is Felt and Relational - as distinct from dissociated fear.
Your voice counts
We welcome your comments and are very interested in your point of view, but we ask that you keep them relevant to the article, that they be civil and without commercial links. All comments are moderated prior to being published. We reserve the right to edit or not publish comments that we consider abusive or offensive.
There is extra content here from a third party provider. You will be unable to see this content unless you agree to allow Content Cookies. Cookie Preferences