The first covid-19 vaccine in the world, from BioNTech & Pfizer, has been released for mass vaccination of the public - in the UK. Outwardly, given the publication of Phase 3 trials in the peer reviewed literature, many may feel that everything they need to know if they are to give their informed consent for vaccination is already known - and is in the public domain.
This simply isn't true.
That is why we've seen fit to create a tracker that can be used to evaluate vaccine transparency for individual vaccines, in specific countries.
In launching the tracker, Transvac, and placing it in the public domain to allow others to engage with the interactive algorithm (in Excel), we hope to be able draw attention to the continued withholding of data that we consider to be essential for properly informed consent.
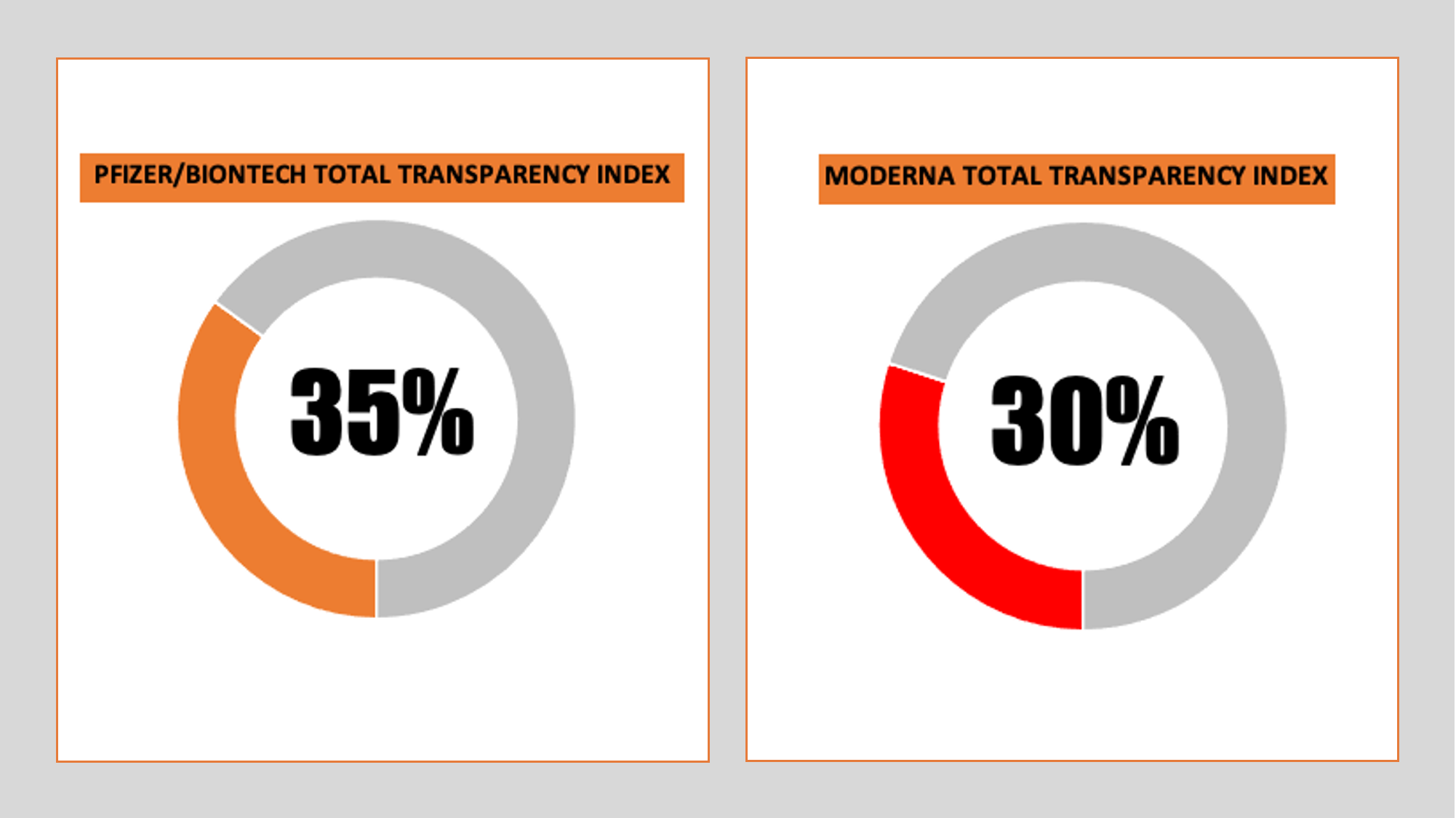
We have initially evaluated with Transvac two vaccines: the BioNTech/Pfizer vaccine in the UK, and the Moderna vaccine in the US. The results showed there is a long way to go before transparency can be declared, the former yielding a 'transparency index' of just 35%, marginally better than Moderna's 30%.
We issued a press release this morning (below) to publicise the new vaccine transparency tool.
PRESS RELEASE
For immediate release
17 December 2020
Newly launched vaccination transparency tool yields ‘transparency index’ of 35% or less for Pfizer and Moderna vaccines
The Alliance for Natural Heath International, a UK-based, internationally active, non-profit campaign, research and education organisation, has released into the public domain an open source vaccine transparency tool, TransVac, that allows comparison of the relative degree of vaccine transparency relating to citizen-facing information for the new generation of covid vaccines.
Using the new tool, Pfizer/BioNTech’s vaccine, currently being administered to the UK public, was found to have a ‘transparency index’ of just 35%. The Moderna vaccine, destined for imminent release in the US, fared even worse with an index of just 30%.
In April, together with colleagues at the British Society for Ecological Medicine (BSEM), the Alliance for Natural Health (ANH) International published an open letter to Matt Hancock, the UK Minister for Health and Social Care, calling for vaccine transparency prior to roll-out of synthetic biology covid vaccines in what is described as the “biggest vaccination drive in British history”.
Given it is crunch time for an increasing number of Britons, and will be soon for US citizens, and others around the world, the vaccine transparency tool was developed to help citizens understand more about the nature and availability of information required for properly informed consent. The tool uses an algorithm to determine compliance with the ten criteria outlined by the ANH and the BSEM in their vaccine transparency manifesto issued earlier this year.
Transvac generated a transparency index for the Pfizer/BioNTech vaccine in the UK of just 35%, with the index varying between 0% and a maximum of 68% for the 10 different criteria. That was marginally greater than the 30% found, using identical criteria, for the Moderna vaccine in the USA, which is expecting an imminent greenlight from the US Food and Drug Administration (FDA).
The results suggest that information required by citizens on which to be able to give informed consent is severely limited, and more opaque than it is transparent.
ANH executive and scientific director Rob Verkerk PhD said,
“We’ve developed the Transvac tool to help people understand what they should be asking health professionals and authorities if they wish to exercise properly informed consent, which is a legal right in most parts of the world for any medical intervention. Historically, vaccine transparency has been poor, and given the synthetic biology platforms and fast-track development timetables for the new generation of covid vaccines, a high degree of transparency is more important than ever.”
Although both the Pfizer/BioNTech and Moderna vaccines use the same mRNA platform, one of the main differences in transparency between the two vaccines is that the lipid nanoparticle (LNP) ingredients used by Moderna, unlike Pfizer, have yet to be released to the public. This prevents any independent scientific risk assessment. In neither case have the amounts of the LNP adjuvants or their specific physico-chemical properties been released. It has been well established that nanoparticle delivery can greatly alter—and increase—the intrinsic toxicity of nanoparticles compared with the same ingredients in non-nano form. It is therefore unsurprising that “Moderna warned that they cannot be sure their LNP’s will not have adverse effects.”
The ANH is calling on the public, independent scientists, elected representatives and health professionals to engage with the open source Transvac tool. Most of the 10 criteria are subdivided into 4 to 6 sub-components and as more information materialises, the indices will change.
Dr Verkerk said that the ANH has been inundated by members of the public who say they are hesitant to receive covid vaccines because of a lack of information, and added:
“The public is often blamed for vaccine hesitancy. But accountability lies more with the vaccine makers and regulators for information that generates confidence. Transvac provides transparency over the nature of information that the public should have access to if the right to informed consent is to be respected during the forthcoming mass vaccination programs.”
The Transvac Dashboard
Table 1: Data source: ANH-Intl Transvac vaccine transparency tool
|
||||||||
| Source data: 16 December 2020 | ||||||||
| CRITERIA | Pfizer/ BioNTech % |
Moderna |
||||||
| UK | US | |||||||
| 1 | Full disclosure of all raw data from safety studies of commercial Covid-19 vaccines | 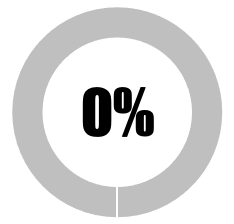 |
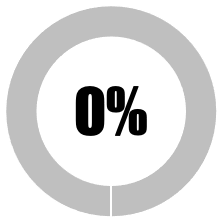 |
|||||
| No raw data in the public domain | 0 | 0 | ||||||
| 1-25% raw data in public domain | 0 | 0 | ||||||
| ≥25%%<50% of raw data in public domain | 0 | 0 | ||||||
| ≥ 50% < 99% raw data in public domain | 0 | 0 | ||||||
| ≥ 99% of raw data in the public domain | 0 | 0 | ||||||
| SUB-TOTAL | 0 | 0 | ||||||
| 2 | Transparency in relation to safety and efficacy studies | 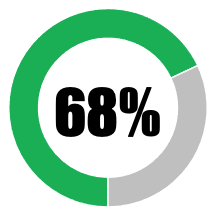 |
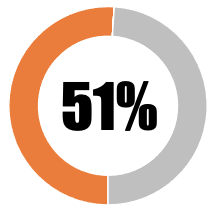 |
|||||
| No peer review publication of any safety or efficacy data | 0 | 0 | ||||||
| Peer review publication of safety and efficacy data (partial or summary data) from Phase 1/2 trials | 17 | 17 | ||||||
| Peer review publication of safety and efficacy data (complete data) from Phase 1/2 trials | 17 | 17 | ||||||
| Press release of partial topline data from Phase 3 trials | 17 | 17 | ||||||
| Press release of comprehensive topline data from Phase 3 trials | 0 | 0 | ||||||
| Peer review publication of safety and efficacy data (partial or summary data) from Phase 3 trials | 17 | 0 | ||||||
| Peer review publication of safety and efficacy data (complete data) from Phase 3 trials | 0 | 0 | ||||||
| SUB-TOTAL | 68 | 51 | ||||||
| 3 | Transparency over the type of platform used for commercial vaccines | 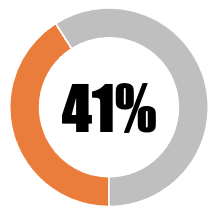 |
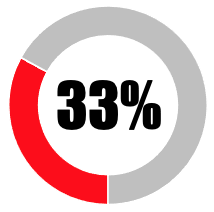 |
|||||
| Platform unknown | 0 | 0 | ||||||
| Platform type declared by WHO or national regulator | 33 | 33 | ||||||
| Platform declared in citizen facing promotion of mass vaccination programme | 33 | 0 | ||||||
| Synthetic biology terminology (or related nomenclature e.g. synthetic, gene edited) declared in citizen facing promotion of mass vaccination programme | 0 | 0 | ||||||
| No declaration in citizen facing promotion of absence of previous use of novel vaccine technology in mass vaccination programmes | -25 | 0 | ||||||
| SUB-TOTAL | 41 | 33 | ||||||
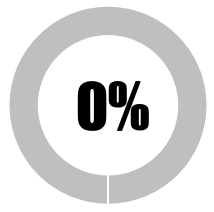
| 4 | Conduct and transparency of studies to elucidate any risks associated with adjuvants as distinct from antigens |  |
 |
|||||
| No published (including peer reviewed) safety studies on adjuvants used in same format as vaccine | 0 | 0 | ||||||
| Third party peer review safety studies for adjuvants that are bioequivalent | 0 | 0 | ||||||
| Raw data for third party peer review safety studies (for adjuvants that are bioequivalent) available in public domain | 0 | 0 | ||||||
| Product specific safety studies published in peer review | 0 | 0 | ||||||
| Raw data for product specific safety studies available in public domain | 0 | 0 | ||||||
| SUB-TOTAL | 0 | 0 | ||||||
| 5 | Transparency in relation to vaccine composition | 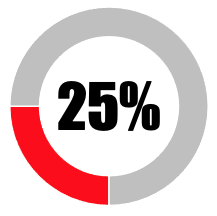 |
 |
|||||
| No declaration by manufacturer or regulator approving emergency use of all ingredients present in vaccine | 0 | 0 | ||||||
| Declaration by manufacturer or regulator approving emergency use of all ingredients present in vaccine | 25 | 0 | ||||||
| Declaration by manufacturer or regulator approving emergency use of amounts of all ingredients present in vaccine | 0 | 0 | ||||||
| Declaration by manufacturer or regulator approving emergency use of quality assurance tests for any contaminants in vaccine | 0 | 0 | ||||||
| Declaration by manufacturer or regulator approving emergency use of physico-chemical characteristics (including of any nanoparticles) of all ingredients present in vaccine | 0 | 0 | ||||||
| SUB-TOTAL | 25 | 0 | ||||||
| 6 | Full disclosure of cases and potential cases of vaccine injury (= adverse events, whether short- or long-term, whether from active constituents, adjuvants or contaminants, intentionally or unintentionally added to the vaccine) |  |
 |
|||||
| No data on nature or severity of data from Phase 3 trial or commercial use in public domain | 0 | 0 | ||||||
| Summary data from Phase 3 trial safety endpoints available in public domain | 25 | 25 | ||||||
| National authority has published, or declared its intention to publish in the public domain, postmarketing surveillance data relating to safety/adverse events | 0 | 0 | ||||||
| National register established for recording vacccine adverse reactions | 25 | 25 | ||||||
| Primary care physicians have been formerly notified by appropriate health authorities to add patients presenting with adverse reactions to national register | 0 | 0 | ||||||
| SUB-TOTAL | 50 | 50 | ||||||
| 7 | The Government must clarify eligibility and criteria for no-fault vaccine injury payments for Covid-19 vaccines | 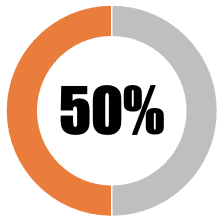 |
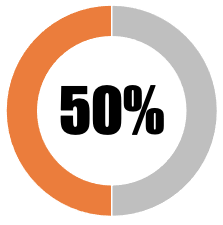 |
|||||
| The national government has provided no public clarification of eligibility and criteria for no-fault vaccine injury compensation | 0 | 0 | ||||||
| The national government has placed in the public domain some, but inadequate, information about eligibility and criteria for no-fault vaccine injury compensation | 50 | 50 | ||||||
| The national government has placed in the public domain comprehensive information about eligibility and criteria for no-fault vaccine injury compensation | 0 | 0 | ||||||
| SUB-TOTAL | 50 | 50 | ||||||
| 8 | The Government must clarify indemnity offered to vaccine manufacturers | 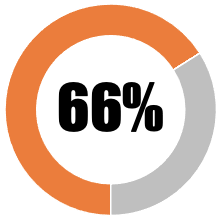 |
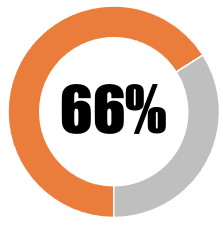 |
|||||
| No information provided to the public about government indemnity of vaccine industry against liability in the event of 'no-fault' vaccine-injury | 0 | 0 | ||||||
| Some, but grossly inadequate information, provided to the public about government indemnity of vaccine industry against liability in the event of 'no-fault' vaccine-injury | 33 | 33 | ||||||
| Some, but nevertheless inadequate information, provided to the public about government indemnity of vaccine industry against liability in the event of 'no-fault' vaccine-injury | 33 | 33 | ||||||
| Comprehensive information, provided to the public about government indemnity of vaccine industry against liability in the event of 'no-fault' vaccine-injury | 0 | 0 | ||||||
| SUB-TOTAL | 66 | 66 | ||||||
| 9 | The public must be informed of the extent of naturally-acquired immunity prior to public release of Covid-19 vaccines |  |
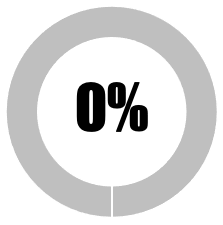 |
|||||
| No publicly accessible data on main national covid-19 portal on proportion of national population with naturally-acquired immunity (antibodies and/or T-cells) | 0 | 0 | ||||||
| Some, but inadequate, publicly accessible data on main national covid-19 portal on proportion of national population with naturally-acquired immunity (antibodies and/or T-cells) | 0 | 0 | ||||||
| Comprehensive publicly accessible data on main national covid-19 portal on proportion of national population with naturally-acquired immunity (antibodies and/or T-cells) | 0 | 0 | ||||||
| Regional publicly accessible data on main national covid-19 portal on proportion of regional population with naturally-acquired immunity: antibodies only | 0 | 0 | ||||||
| Regional publicly accessible data on main national covid-19 portal on proportion of regional population with naturally-acquired immunity: antibodies and T-cell responses | 0 | 0 | ||||||
| Members of the public given the option to have immunity status tested prior to vaccination | 0 | 0 | ||||||
| SUB-TOTAL | 0 | 0 | ||||||
| 10 | Any decision to mandate Covid-19 vaccines or limit freedoms of the unvaccinated must be democratic | 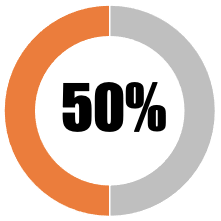 |
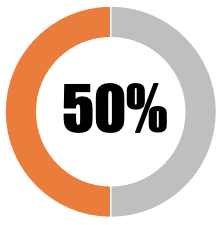 |
|||||
| Government or national authority has formally deliberated mandating covid vaccines or limiting freedoms of the unvaccinated but has not engaged with the democratic process (elected representatives) | 0 | 0 | ||||||
| Government or national authority has formally deliberated mandating covid vaccines or limiting freedoms of the unvaccinated but has not engaged with any aspect of the democratic process (e.g. elected representatives and public consultation) | 25 | 25 | ||||||
| Government or national authority has formally deliberated mandating covid vaccines or limiting freedoms of the unvaccinated with input from elected representatives | 25 | 25 | ||||||
| Government or national authority has deliberated mandating covid vaccines or limiting freedoms of the unvaccinated and has included input from a public consultation | 0 | 0 | ||||||
| Government or national authority has deliberated mandating covid vaccines or limiting freedoms of the unvaccinated final decision will be made on the basis of a democratic vote by elected representatives | 0 | 0 | ||||||
| SUB-TOTAL | 50 | 50 | ||||||
| TOTAL TRANSPARENCY INDEX | 35 | 30 | ||||||







Comments
your voice counts
18 December 2020 at 9:03 am
Please describe how the sub-categories are to be weighted in importance for calculating the overall percentages for each category. Section 8 appears to have two identical sub-categories. It is also unclear how the Vaccine Transparency tool is to be populated with data and how the data is to be disseminated to users. Overall, the intention looks good but the devil's in the detail!
18 December 2020 at 11:21 am
Sincere appreciation for this TRANSVAC DASHBOARD.
One begs the Public to heed this information, leading to a decision to support their bodies with Supplements, Exercise & healthy non-GMO food ONLY.
One thinks of the word "collusion" reading these figures. One lot from the Moderna// Gates totally owned Factory, who have never had any success in this field before the purchase by B Gates who would have purchased Moderna cheaply, then easily installs HIS sycophants into controlling positions (unlike the Cochrane Collaboration takeover). Pfizer, that is a name that is up there in the corrupt Big Pharma world, say no more.
An insult to the public that there are so many zero's (0) in this list and serves to confirm their deepest suspicions.
Your list compounds decision-making to stay in Italy for a total of 2 years, until the panic has settled, and (hopefully) truth is disseminated in the public area - if able too, and the public becomes aware of the damage to their Auto Immune systems by these DNA permanent, life-changing Vaccines.
Auto Immune systems which do/did such a wonderful job for us, until corrupt Science/CIA/ etc., utter greed, power, control, paid infiltration of a variety of organisations by monied players, and those reading from the prescribed Script.
All the illnesses that we have seen over the last 30+ years, that were rarely present in our society before, and in our children, that we now see because of the CORRUPTION and greed in Government bodies and their ilk, we used to be able to have some semblance of trust in. And hopefully the removal of unelected glitterati, hubristic self-elected groups that think their usually most dishonestly made cash, gives them a right to dictate others’ lives.
People NEED to stop supporting the Amazons, Facebooks, Walmart’s etc., of this World, its one way to send an extraordinarily strong message to these Psychopaths. They understand anything to do with the bottom line, that they have used to seduce so many into using their platforms. PLEASE!!!
Shall there be updates on this information in the future? If any!
22 December 2020 at 5:00 pm
I Like the name Transvac as it appears one ingredient (if one can call it that) is reported to be CYP19A1
Quote; Aromatase is a key enzyme in estrogen production. It converts androstenedione and testosterone to estrone and estradiol, respectively [2].
There is almost total transparency on these vaxterminations so I am a conspiracy theorist...
30 January 2021 at 3:41 pm
Is there a similar tool specifically for the multiplier used in PCR tests?
And criteria used for positive 'cases'?
I see plenty on gross figures, but nothing on the underlying tech.
Your voice counts
We welcome your comments and are very interested in your point of view, but we ask that you keep them relevant to the article, that they be civil and without commercial links. All comments are moderated prior to being published. We reserve the right to edit or not publish comments that we consider abusive or offensive.
There is extra content here from a third party provider. You will be unable to see this content unless you agree to allow Content Cookies. Cookie Preferences