Content Sections
ANH-Intl’s founder and scientific director gave a keynote presentation at the highly acclaimed Food Integrity conference, once again forced online by current events. The subject was ‘Novel Foods and Trends’ and Dr Verkerk went on to moderate a panel on CBD.
Highlights
Highlights of Dr Verkerk’s keynote are summarised here:
- The UK hones its own risk assessment, risk management and authorisation system for novel foods, relying for the time being on scientific guidance developed in conjunction with the European Food Safety Authority (EFSA)
- Of 139 applications made to the EU to-date, 17 have been for micronutrients, 14 for plant foods, 13 for human milk oligosaccharides (HMOs), 12 for plant extracts and 12 for edible insects (see Fig. 1)
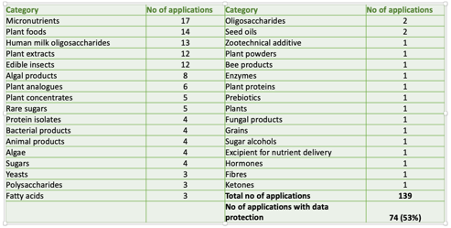
Figure 1. Summary of novel food applications in the European Union up to April 21, 2021 (Data source: European Commission).
- CBD has been the most rapidly growing natural product category in recent times, with CBD becoming a common ingredient in health, cosmetic and vaping products
- EFSA’s decision to regard all natural and synthetic cannabinoids as novel foods or ingredients in January 2019 put a massive brake on the industry, so impacting innovation and consumer choice. This was despite natural cannabinoids or cannabimimetics having been consumed safely in a variety of foods and herbal products including hemp oil, Echinacea, black truffles, and cacao for decades, albeit in smaller amounts
- Cannabinoids are also generated internally and interact with the endocannabinoid system with CB1 and CB2 receptors widely distributed through our bodies. We produce cannabinoids internally (endocannabinoids) when we engage in certain rhythmic exercises like walking, jogging, cycling or swimming and they appear to activate the senses, reduce negative stress responses and improve cellular communication
- While some EU countries like France were starting to crackdown on CBD claiming it was narcotic, despite it having no psychoactivity, a ruling by the European Court in May 2020 (Case C-663/18) found that the French decree of 22 August 1990, which limited the cultivation, industrialisation and marketing of hemp solely to fibre and seeds, amounted to a restriction that was not in accordance with EU law. That opened the door, up until....
- The European Commission paused the processing of applications for novel foods in July 2020 because it believed, along with a number of EU member state governments, that CBD, as an extract of cannabis, should be regarded as a narcotic substance based on the UN Single Convention on Narcotic Drugs, 1961
- In December 2020, the UN Commission on Narcotic Drugs did a U-turn and removed cannabis from the narcotics schedule, 59 years after it had been added. It also stated that CBD extracts containing less than 2% of the psychotropic component THC should not be subject to international controls
- The UK has potentially become a safe harbour for CBD products, assuming novel food applications for CBD are accepted. It upheld that products sold before 14 February 2020 with validated applications submitted before 31 March 2021 should be able to be legally on sale until authorisation is determined
- So far, applications for only 23 products have been validated by the Food Standards Agency, the UK authority responsible for foods. These were submitted by just 3 companies, Chanelle McCoy Health (Ireland), Cannabis Pharma (Czech) and Health Innovations (UK). Up to 1000 applications are thought to have been submitted prior to the 31 March 2021 deadline
- A major conundrum remains for cannabinoids in the UK and EU, especially around how to reduce trade barriers while maintaining consumer safety and trust, while also encouraging smaller businesses that have for years been the bedrock for innovation in the natural products industry
- Applications for synthetic CBD (4 of which are already approved) and CBD isolates will be considerably more straight forward than those for full spectrum extracts, given the complexity of the latter. This creates a bias towards forms that deviate more from the natural forms of cannabinoids to which humans have been exposed during our evolution.
Panel discussion – The Rise of Cannabis, CBD and Hemp
Dr Verkerk then went on to moderate a panel of experts, comprised of the following 4 experts:
- Dr Prof Hans Verhagen, Owner and founder, Food Safety & Nutrition Consultancy, ex- NDA panel member, EFSA
- Dr Parveen Bhatarah, Regulatory and Compliance Lead, Association for the Cannabinoid Industry (ACI)
- Lorenza Romanese, Managing Director, European Industrial Hemp Association (EIHA)
- Dr Ignazio Garaguso, RSL, PerkinElmer
Like ourselves at ANH-Intl, we consider the view of the European authorities that all cannabinoids are novel, to be wrong, a view also taken by Lorenza Romanese of the EIHA. This definition should be limited to synthetic cannabinoids and CBD and other cannabinoid isolates, that are not found in their isolated form in nature.
The CBD industry has not taken the novel food challenges for cannabinoids lying down. Two important developments that may have a big impact on the long-term availability of CBD products in the UK and subsequently in the rest of Europe are as follows:
- The ACI based in the UK has created a consortium that has submitted a master application for CBD to the UK’s FSA in February
- The 300 or so members of the Brussels-based EIHA have also formed a consortium of hemp farmers, producers and traders who have also submitted applications to the FSA, both for a natural CBD isolate and for a full spectrum extract.
Footnote
The Food Integrity conference is organised by Russell Publishing, publishers of New Food magazine. ANH-Intl’s Robert Verkerk PhD is a member of the New Food’s advisory board.








Comments
your voice counts
29 April 2021 at 7:05 pm
I think this obsession around CBD being a cure all needs much more balance and realisation that it is not tolerated by everyone and doesn't work as it claims for everyone. We have to go back to the unique physiology of each individual.
The EU and UK laws do need to be careful because people misunderstand that natural substances can also be harmful.
I always err on the side of caution in any of these debates and decisions because I have experienced within my own health that CBD actually causes me more harm so I steer away from it. We must always remember that whatever any substance is given it affects the liver for good or for ill. You may like to remember that back in the time of Leonardo Da Vinci the drug of choice was the spice Nutmeg (sniffed like cocaine) and you can read about its narcotic properties here https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/nutmeg and here https://www.healthline.com/health/high-on-nutmeg
Everything we ingest nutritionally as food or as supplements has both good effects and not so good and is dependent on the persons own energetic makeup as to how it works. Long term use of CBD in any form will have the same effect as using any drug on the liver.
The Natural Products industry does need some regulation, and I feel the fight should be in better sensitive testing that can then be presented to mainstream science without argument. If evidence based medicine takes over as it is doing currently with poor testing mechanisms, then the fight for natural products will become tougher and tougher and censorship more prevalent until we get to the point like Greece and other EU countries where nutrition can only be sold by Pharmacies or prescribed by hospital dietician's or sports nutritionists.
At the moment the UK is still in quite a privileged position that we have a strong natural nutrition and herbal voice and are allowed to practice, in other EU countries this is not so easy.
Therefore a balanced discussion and regulation on CBD is needed.
Your voice counts
We welcome your comments and are very interested in your point of view, but we ask that you keep them relevant to the article, that they be civil and without commercial links. All comments are moderated prior to being published. We reserve the right to edit or not publish comments that we consider abusive or offensive.
There is extra content here from a third party provider. You will be unable to see this content unless you agree to allow Content Cookies. Cookie Preferences