Content Sections
- ● Risk assessment: the use of bad science for the benefit of the multinationals
- ● Applying the risk assessment models used for toxic chemicals to nutrients
- ● What’s wrong with conventional risk assessment approaches?
- ● ANH summary of problems with conventional risk assessment as applied to nutrients
- ● Brief synopsis of both Dr Verkerk's papers critiquing European approaches to risk analysis
- ● Related pages
Risk assessment: the use of bad science for the benefit of the multinationals
'Risk assessment' is not a sexy or fashionable term. But it's the mechanism that is being used by regulators around the world, and decision-makers in Codex, to limit our freedoms in natural healthcare.
Risk assessment is used in three main ways:
- To ban or limit ingredients allowed in natural health products
- To limit maximum dosages of nutrients and phytochemicals
- To limit what we can say or write about, particularly as it relates to health benefits of particular products.
The purpose? To supposedly protect us from hurting ourselves. It's all very plausible until you realise that there's virtually no evidence that anyone is actually doing themselves any harm by taking these products. Quite the contrary—people are making themselves healthier and healthier....and therein lies the real threat!
Risk assessment science is being increasingly used in virtually all walks of life—ranging from the safety of motor vehicles, to the safety of schools and the workplace. This new branch of science—considered by some to be more of a quasi-science give the amount of subjectivity it often uses—has been applied to food safety for some time. However its main application has been to ensure that unsafe levels of pathogens and toxins within foods, such as E.coli and other bacteria, food additives, preservatives and pesticide residues, are avoided. The fact that many harmful additives and pesticides are still common in foods is testament to how risk assessment has been manipulated to the benefit of those interests which profit from chemically contaminated foods.
To find out how flawed risk assessment is at risk of being used to dumb down European supplements through the EU Food Supplements Directive—and eventually, globally, see the ANH's Codex campaign and our Freedom of Health Choice campaign. This will happen unless the authorities respond to common sense, good science—as well as the will of hundreds of thousands of people around the world.
Get involved in the ANH campaign and help future generations have the option of natural health.

WRONG TOOL FOR THE JOB? Should we apply the same risk assessment methods to assess these pollution agents as for the nutrients in food?
Applying the risk assessment models used for toxic chemicals to nutrients
But now risk assessment science is being applied to nutrients and botanicals used for health promotion—yet this science completely avoids any consideration of the benefits conferred.
In Europe, the European Commission, the European Food Safety Authority and the Federal Institute for Risk Assessment (BfR, in Germany) are the key instigators of risk assessment for nutrients, while at an international level, it is the World Health Organization and the Codex Alimentarius that are the main protagonists of this approach.
What’s wrong with conventional risk assessment approaches?
In 2002, the ANH issued a detailed consultation to the UK Expert Group on Vitamins and Minerals (EVM), which heavily criticised the EVM’s approach to risk assessment.
In 2003, the ANH submitted a detailed critique of conventional risk assessment as applied to nutrients in its consultation response to the FAO/WHO nutrient risk assessment project.
Building on ANH’s work, in 2005, Dr Jaap Hanekamp and Professor Alt Bast of the HAN (Heidelberg Appeal Nederland) Foundation in the Netherlands commenced work on deconstructing the problems associated with risk assessment and the European regulatory model as applied to natural health products.
The following are key outputs from their research, published in 2006 and 2007:
Hanekamp(2006) on the precautionary principle in the context of the EU Food Supplements Directive.
Hanekamp & Bast (2007): food supplements within a precautionary context.
Hanekamp & Bast (2007): food supplements and fortified foods—and the the EC's patriarchal precautionary perspective on public health.
ANH summary of problems with conventional risk assessment as applied to nutrients
An excerpt from Verkerk/Hickey 2009:
1. The Upper Limit (UL) is equally applicable to all healthy life-stages and population groups. However, susceptibility is clearly not equivalent across all life-stages and population groups, as recognised by the FAO/WHO expert group (2006).
2. The ‘critical adverse health effect’, selected as the most sensitive adverse effect, is generally applied equally to all population groups. This generalisation may be inappropriate for significant sub-populations. The ‘critical adverse health effect’ in question will not be experienced by those with higher tolerance (common, for example, in the case of vitamin C), those who have adapted to higher intakes of the nutrient (common, for example, with vitamin B3) or those who consume the nutrients in different nutritional forms or in different matrices (bioavailability and formulation may drastically affect absorption rates by the body).
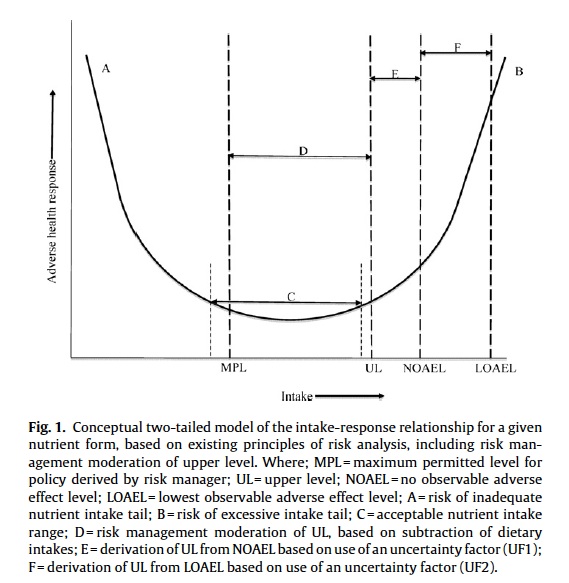
3. The ULs are based on a single ‘critical adverse health effect’ which is deemed the most sensitive effect, occurring at the lowest level of intake. The adverse effect may be mild, transient, reversible and inconsequential as compared with the benefit derived. No account is taken of adverse health effects that may be more serious, or that overlap with benefits, which may occur at substantially higher levels of intakes. While it is usual to consider a single two-tailed curve to describe the intake-response relationship (Figure 1), a more realistic representation would reflect individual (multiple) intake-response curves, each specific to a given metabolic system influenced by the nutrient in question.
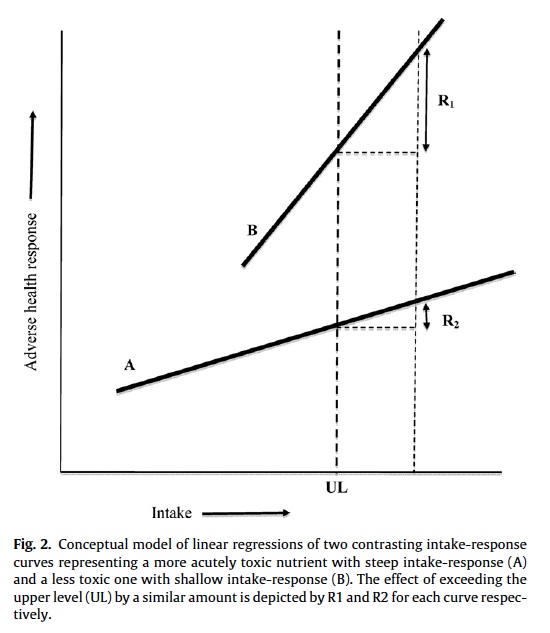
4. It is assumed that the risk of exceeding the UL incurs equivalent risk irrespective of the nutrient considered. Assuming differing intake-response slopes (e.g., low for calcium, high for selenium), as well as the different nature, severity and, in most cases, reversibility of the adverse effects, the risk of exceeding the UL by a given amount has greatly varying consequences (Figure 2). At the very least, current models of risk assessment should be adjusted by way of a correction or weighting factor, which takes into account the slope of the intake-response curve as well as the nature, severity and reversibility of the adverse effect.
5. The UL or Guidance Level (GL) (Table 1) is based on the most hazardous member of a given nutrient group. It effectively applies the precautionary principle disproportionately (Hanekamp and Bast, 2007a, 2007b) to safer members of the same nutrient group. If the UL is used as the basis for risk management, it is likely to cause disproportionate effects on safer nutrients that may lead to sub-optimal intake of the given nutrient. Different hazard profiles for members of the same nutrient group are largely a function of differences in pharmacokinetics, pharmacodynamics, mechanism of action, bioavailability and metabolic conversion, as well as differences in safety/hazard/benefit of any bound compounds, substances or biological materials. Well recognised examples of differences in risk or hazard (greater less) among related nutrients, all (except niacin-bound chromium) included in Annex II of EC Regulation No 1170/2009, which amends Directive 2002/46/EC on food supplements, are:
- Vitamin A: retinol—carotene
- Vitamin B3: nicotinic acid—niacinamide—inositol hexanicotinate
- Vitamin C: L-ascorbic acid—potassium ascorbate
- Vitamin D: ergocalciferol (D2)—cholecalciferol (D3)
- Vitamin E: alpha-tocopherol—gamma-tocopherol (predominant isomer in commonly used ‘mixed tocopherols’ ingredients)
- Folate: folic acid—polyglutamate folate (e.g., calcium-L-methylfolate)
- Iron: iron sulphate—ferrous bisglycinate
- Chromium: chromium chlorid—niacin-bound chromium
- Selenium: selenious acid—selenomethionine
Of the above, while risk assessors normally take account of differences between the flushing effect (which they consider as an adverse health effect) of two different forms of vitamin B3, namely nicotinic acid and niacinamide, they normally do not take into account the ‘flush-free’ attribute of inositol hexanicotinate. For most other nutrients, the UL is based on the most form of a given nutrient most likely to trigger an adverse health effect. By example, for iron, the adverse effect caused by iron sulphate (typically the form administered medically for the treatment of anaemia) is applied to all forms of iron, so acting disproportionately on safer nutrient forms, such as iron bisglycinate (known to many consumers as ‘gentle iron’), which is not associated with any adverse effects at normal supplemental doses.
Where there are clear differences in safety (and benefit) profile between different molecular forms of a nutrient within a given group, separate ULs and, where appropriate, MPLs, should be determined consistent with appropriate scientific standards (Brunton et al, 2005). These relationships may be better understood, and therefore be managed better through regulation, by using a common currency for risk and benefit assessment, such as a quality of life statistic (EFSA, 2007).
6. Surrogates for ULs (e.g., Guidance Levels (GLs) as set by the EVM) are based on inadequate data sets, yet are treated by risk managers in the same way as ULs that are based on more complete data. This approach is not scientifically robust and may result in setting excessively precautionary MPLs, such as those determined by the BfR (Table 1), especially in cases where ULs are lacking. Where there are insufficient data to determine ULs with a high degree of confidence and where there is no evidence of toxicity of a nutrient at even very high intakes (e.g., vitamins B1, B2, B12), it would be more rational scientifically to use the highest observed intake (HOI) levels (as determined from food and supplementary sources) as an appropriate ‘risk management level’. This approach is suggested by the FAO/WHO expert group (2006). The subtraction of highest mean intakes from these levels is unjustified and will lead to unnecessary restriction of intakes in supplements, functional and fortified foods.
7. The UL is determined on the basis of a given use-pattern, usually the consumption of the daily amount in a single dose either with or without conventional food. Where the nutrient has a short half-life in the bloodstream and is rapidly metabolised (e.g., vitamin C; Hickey et al, 2008), such ULs are not applicable to different use-patterns, such as the consumption of higher amounts in divided doses. Different use-patterns may be countenanced by risk managers on the basis that the directions for use of such products are stated accordingly (e.g., maximum dosage, 1000 mg every 3h).
8. The data on which ULs are based are limited in type and do not generally include evidence from clinical nutrition practice or medical records. They also do not include data from government adverse event reports. One of the most comprehensive adverse event data sets, that includes acute, chronic and acute-on-chronic exposures, is the National Poison Data System (NPDS) produced through compilation of data from the national network of Poison Control Centers in the USA (e.g., NPDS, 2008). The data are published annually in the journal Clinical Toxicology. These data provide invaluable information on the relative hazard of different nutrients and should be used to inform prioritisation of nutrient risk management. A summary of data relating to vitamin exposures from the last 10 years of the NPDS is given in Table 2.
9. Uncertainty factors (UFs) used in the determination of ULs are often arbitrary and subjective. If a No Observable Adverse Effect Level (NOAEL) is used, there should be no requirement to employ a UF in the case of nutrients that have a low risk profile; the NOAEL specifies that there is no known risk of adverse health effects in the case of exposures up to this level. This approach is appropriate and necessarily differs from that used in classical risk assessment for environmental chemicals for which there is no risk of inadequacy and no benefits derived from higher levels of intake. However, in the unique case of nutrients, use of a UF to moderate the NOAEL could, following risk management, increase the risk of inadequacy and prevent some population groups from obtaining optimal intakes.
10. Risk assessment models are being increasingly accepted as valid by government authorities with no apparent attempt to validate them against empirical data. The assumption that risk increases in a dose-dependent manner is a foundational principle of toxicology (Brunton et al, 2005). However, in the case of nutrients, while risk and benefit cannot be validly compared without a common currency (EFSA, 2007), it is not possible to ignore the beneficial effects of higher intakes, e.g., of vitamin C (Douglas et al, 2007), and vitamin D (Vieth, 2007; Grant et al, 2009). In particular cases (e.g., vitamin A; FAO/WHO, 2006), there may be an overlap between risk and benefit, implying that risk management that prevents consumer access to intake levels that may give rise to a risk of an adverse health effect, may also prevent the same (or different) sub-populations gaining benefit.
Brief synopsis of both Dr Verkerk's papers critiquing European approaches to risk analysis
Legally binding maximum intake levels for nutrients, currently in development by the European Union (EU), are fundamentally flawed and could deprive consumers of major health benefits.[1,2]
The vital role of diet and nutrition in the prevention of disease, especially chronic disease, is gaining recognition. Increasing numbers of people throughout the industrialised world supplement their diet with vitamins and minerals to maintain or improve their health. To ensure public safety, the EU is assessing the risks associated with nutrients in order to generate upper levels (ULs) of daily intake (“the maximum level of habitual intake from all sources of a nutrient...judged to be unlikely to lead to adverse health effects”). The aim is to develop consistent regulation across EU member states, defining both lower and upper limits – or maximum permitted levels (MPLs) – of nutrients consumed in supplements and fortified foods.
There are serious problems with the process as it stands.[1,2] The methods of risk assessment being used to develop EU-wide laws have not been scientifically validated; and the chosen models are based on simple formulae that ignore complex interactions between nutrients, individuals and populations.[1] The EU model proposes a single UL for each nutrient. This concept has several drawbacks, including:[1]
- ULs vary in individuals at different stages of life, and in different populations
- Adverse effects of relevance when calculating ULs occur differently across populations
- Adverse effects may be unimportant relative to the benefits derived from the nutrient
- It is assumed, incorrectly, that an identical risk of exceeding the UL applies to all nutrients
- Where nutrients naturally occur in different forms, the UL is based on the most hazardous form.
Additionally, the methods used to produce MPLs pose several issues, such as:[1]
- No consideration of different molecular forms of nutrients when calculating MPLs
- Calculating risk based on the effect of the most hazardous form of a nutrient on the most sensitive populations
- Ignoring, for convenience, differences in nutrient intake between EU member states
- Calculating nutrient intake without considering that consumers of fortified foods and supplements are different groups.
This imperfect and highly cautious approach leads to various problems. In essence, these models assume a 'safe intake level', below which lies the risk of nutrient insufficiency and above which lies the risk of excess. In fact, numerous adverse effects and benefits can occur over a wide range of intake of any nutrient. An improved model is suggested that takes this into account, and which proposes a 'zone of overlap' between risks and benefits.[2]
Risk–benefit assessment was applied to four nutrients: folate (vitamin B9), niacin (vitamin B3), selenium and fluoride. A common finding was that doses in excess of the EU-defined ULs caused health benefits in most people, as well as risks to sensitive populations. This was the case for all of the nutrients studied except selenium, and is probably the norm.[2]
Rather than risk assessment, a more rational approach to regulation of nutrient dosage in the EU would apply risk–benefit analysis, possibly employing the 'zone of overlap' concept. Statutory restriction of nutrient dosages should be delayed until appropriate risk–benefit models can be developed, validated and adopted.[1,2]
[1] Verkerk RHJ, Hickey S. A critique of prevailing approaches to nutrient risk analysis pertaining to food supplements with specific reference to the European Union. Toxicology 2010;278:17-26.
[2] Verkerk RHJ. The paradox of overlapping micronutrient risks and benefits obligates risk/benefit analysis. Toxicology 2010;278:27-38.
Related pages
25/01/18 Germany:giving (vitamins) with one hand, taking with the other
25/01/18 Can tech help improve our self-care?
17/01/18 The Founder’s Blog: Attenborough, Cox and roadblocks to natural medicine
17/01/18 In sickness and health: is the UK NHS at breaking point?
20/12/17 ANH-Intl Special Report: Vitamin B6 - “It’s the Form, stupid”
13/12/17 Germany’s magnesium clampdown
27/04/17 Should we still be worshipping at the altar of peer-reviewed science?
14/12/16 Fear of fat: the witch hunt against science-based nutrition
25/05/16 The Royal Society whitewash on GM crops
23/09/15 UK government committee side-lines new vitamin D science
24/06/15 Enough evidence to ban world’s no.1 herbicide
03/06/15 Australian mouse study: signal to return to high carb diet?
13/05/15 ANH-Intl Feature: Spanishstudy - do high protein Mediterranean diets kill you quicker?
22/04/15 Vitamin bashing begins in US prior to publication of meta-analysis using old studies
13/01/15 Support for Vitamin D
9th December 2015 Tim Noakes trial
2nd December 2015 Toxxscan crowdfunding launch
4th March 2015 Integrated pancreatic cancer research kicks off as legacy to Jon Lord of Deep Purple
12th February 2015ANH-Intl Feature: Fuel efficiency and the Food4Health Plate
7th January 2015 ‘Cancer is down to bad luck, not genes and lifestyle’ says new study
10th December 2014 Roundup ‘GMO crop’ herbicide toxic to the heart
10th December 2014 Lancet report says it’s untrue that there are ‘no proven treatments’ for Ebola
3rd December 2014 GM crops better for farmers, says German study
3rd December 2014 ANH-Intl Feature: DTC genetic tests from 23andme available in UK
19th November 2014 Do B vitamins help brain ageing?
3rd September 2014 Whistleblowing CDC senior scientist goes public as new evidence is suppressed
13th August 2014 ANH-Intl News Alerts: Week 33, 2014
13th August 2014 Blatant pseudoscience relieves FDA headache for aspirin manufacturers
6th August 2014 Update on the assault on South African natural healthcare
5th August 2014 ANH-Intl News Alerts: Week 32, 2014
16th July 2014 Is the EU preparing to dumb down your supplements?
25th June 2014 Has 4000-year-old Ayurveda tradition reached maturity in the UK?
11th June 2014 Good and bad in revised UK Medical Innovation Bill
28th May 2014 AllTrials is all smiles for Big Pharma: Data transparency and the randomised, controlled trial
30th April 2014 Homoeopathy haters and the RCT religion
27th November 2013 The UK government's obesity strategy is a catalogue of incompetence – at best
13th November 2013 ANH-Intl commissions new approach to determining vitamin safety
6th November 2013 Maintaining myths, corporate science and history repeating itself
23rd October 2013 Saturated fat myth busted in British Medical Journal
2nd October 2013 ANH Book Review: “Dissolving Illusions” by Suzanne Humphries, MD and Roman Bystrianyk
17th September 2013 Why farmer-led research could create a quiet revolution in GM
11th September The battle for Balcombe – and beyond
31st July 2013 ANH-Intl Feature: Fracking: energy but at what cost?
17th July 2013 A tide of evidence washes away flawed fish oil prostate cancer study
10th July 2013 Anti-Science Authority (ASA) provokes rebellion from homeopaths
03rd July 2013 ANH-Intl FEATURE: Clinician effect more powerful than drugs
10th September 2010 Pharma game plan revealed?
8th September 2010 Osteoporosis drugs linked to oesophageal cancer and jaw tissue death
18th August 2010 Would you like chips, tomato sauce and statins with your burger?
4th August 2010 BMJ analysis of calcium studies 'finds' cardiovascular risk
25th June 2010 US professor who faked Pfizer drug research is jailed for 6 months
27th April 2010 ANH petition on Maximum Permitted Levels still open
19th April 2010 ANH Feature: Beware scientism’s onward march!
15th April 2010 The apparent turn around of Professor Ernst
26th March 2010 ANH Press Release: New ANH study says EU vitamin laws must change track
4th March 2010 Oh Dear….Prof Ernst’s research facility faces closure
1st March 2010 Influential US anaesthesia professor faked drug research over 12 years
19th February 2010 European Commission to fund research into complementary medicine
14th January 2010 ANH Press Release: New ANH study exposes flawed nature of EU plans to limit vitamins
18th December 2009 ANH Press Release: 'United we are stronger' - ANH announces merger
9th December 2009 Three EU drug regulators found with unacceptable conflicts of interest
30th November 2009 Congratulations given to the AAHF/ANH
27th November 2009 EC scientific committee ‘unqualified for task’ say international medical doctors group
6th November 2009 French governments monitor food supplements and functional foods for 'side effects'
22nd September 2009 News item - Irish trade group campaigns for truth about supplements
4th August 2009 Facebook used to promote same artificial sweetener in new package
28th June 2009 ANH again challenges science used to justify mass fluoridation of the water supply
22 July 2009 'Unscrupulous health food sellers justify stringent EU health claims regime’, says UK mainstream press
26th June 2009 US censorship of scientifically-backed health claims for selenium
30th March 2009 Plasticiser BPA a step closer to US ban
24th March 2009 Whose quack is louder?
16th September 2008 Dr Rath under fire—is there a silver lining?