Content Sections
- ● Campaign Summary
- ● Where in the world are restrictions most imminent?
- ● March 2012: Health Claims Veto Campaign Update
- ● Canada
- ● United States of America
- ● New Zealand
- ● Why do we need to keep therapeutic natural health products available on the market?
- ● The EU is planning to dumb down our food supplements—imminently!
- ● Making different health choices can change the world.....
Campaign Summary
- As a consequence of moves to harmonise global food laws, concerted attempts to control consumer access to natural heath products are being made by governments and trans-national corporations.
- At a global level the regulatory framework is being developed through the Codex Alimentarius Commission (see our Codex campaign page).
- The system of control essentially moves natural health products from a category of food to products requiring pre-market authorisation, where the authorisation systems being used or proposed are very onerous and so lock out large numbers of products (e.g. around 50% of pre-2004 products in Canada).
- All this is being enacted under the often conflicting guises of ‘consumer protection’ and 'free trade', nearly always creating a situation where big business gets what it wants while our freedom to choose natural health products is dramatically curtailed.
Share information and work with us at the Alliance for Natural Health International (ANH-Intl) so that you can connect the dots, and see why there has never been a more important time for action.
Click here if your computer is wired for sound and you can make the time to listen to a lecture on the subject Natural Health at Risk given Dr Robert Verkerk on 17th June 2008 for Changing Times. It will help you understand why our health choices—particularly natural ones—are so severely threatened.
__________________________________________________________
Where in the world are restrictions most imminent?
The European Union (EU)
Europe is becoming the central platform for global harmonisation of food laws relating to natural health. The ANH-Intl is based in Europe so we are right at the cutting edge. Much of the legal infrastructure that is set to cause major problems downstream has been in place for some time—and is finally beginning to be felt, as key parts of the European regulations and directives are now being implemented.
The EU framework has started to really bite between 2010 and 2011 when—although pro-natural health interests like us have continued to positively influence the process—we have seen:
- massive restrictions on allowed ingredients, including vitamin and mineral forms, as well as botanicals
- further threatened restrictions on maximum permitted doses of vitamins and minerals
- massive restrictions on what can be said about any product with beneficial properties
- imposition of pharmaceutical-type standards, making it difficult for many truly natural products to comply
European laws also form the template being developed by Codex for international harmonisation. If this matters to you, see the ANH Codex campaign and follow our key work on risk assessment.
There are a number of Directives and Regulations in the EU, many of which are in the process of coming into force, that are particularly problematic. Some of the key pieces of European legislation—or shortly to be implemented legislation—along with ANH-Intl's key concerns about them and responses, are detailed in a series of leaflets [issued October 2012]and one-page ANH-Intl Briefing Papers that can be downloaded below:
2012 Downloadable summary information leaflets:
- ‘Food supplements in Europe’ leaflet
- ‘Health claims in Europe’ leaflet
- ‘Plant and herbal ingredients (botanicals)’ in Europe leaflet
- ‘Codex Alimentarius’ leaflet – the international standard-setting body controlling the global food trade
- ‘Practitioners’ leaflet – Building your practice while negotiating the EU regulatory minefield
- Our general leaflet about ANH, with information about out ten campaigns, and how to get involved
Briefing Papers
- EU Food Supplements Directive (FSD)
- Maximum Permitted Levels (MPLs) of vitamins and minerals
- (German translation)
- EU Nutrition and Health Claims Regulation (NHCR)
- EU Traditional Herbal Medicinal Products Directive (THMPD)
- (German translation)
The copius scientific problems associated with the proposed approaches to nutrient risk analysis have now been detailed and elaborated in two scientific papers published in the leading peer-reviewed journal, Toxicology
To read the papers click on the titles below:
Verkerk RH, Hickey S. A critique of prevailing approaches to nutrient risk analysis pertaining to food supplements with specific reference to the European Union.
Verkerk RH. The paradox of overlapping micronutrient risks and benefits obligates risk/benefit analysis.
Earlier leaflets:
- ANH Summary of key challenges to nutritional and botanical forms of natural healthcare, and the ANH's responses to these challenges
- One-page A4 flyer on EU Legislation
- the Good, the Bad and the Ugly of EU Natural Health Regulation.
March 2012: Health Claims Veto Campaign Update

EU health claims battle lost, but war far from over
You have a fundamental right to the information that will help you to understand what you are eating and drinking; to give you the ability to make informed choices; and ultimately, to have control over your health and wellbeing.
We would like to thank everyone who took the time and trouble to write to their MEPs to try to convince them that a veto of the health claims proposal was necessary. So what happens next, you might ask? Watch this space, and we will let you know what our next move should be!
April 2012
There are two key actions that you can help with, one intended for European consumers, the other for food supplement companies. Please sign up to the following, accordingly:
Consumers, please sign the petition (14,899 at the time of writing) at: www.sup.nl/petition
Food supplement companies, please join the signatory list (344 companies at the time of writing) established by the European Health Claims Alliance: www.healthclaimsletter.org
________________________________________________________
Canada
The Canadian government, against the wishes of its its people and its Parliament, brought in a licensing system for 'natural health products' in 2004 which is so onerous, it has already caused a significant loss of products from health stores. It is estimated that some 20,000 products have already been lost.
This system was forced through after a huge public outcry and demonstration of concern about unnecessary restrictions on natural health products in 1997, which culminated in the 'third category' for natural products, keeping them outside of both the food and drugs categories.
But, following the imposition of the Natural Health Products Regulations of 2004, things have been steadily getting worse. Like the European model—the worst of it has yet to be felt—because there is a huge backlog of products being considered for licensing that can legally be sold until the applications are evaluated.
The Canadians have been desperate to get extremely restrictive laws through any which way possible, but thanks to the work of some very concerned citizens Bills C-51, C-52 and C-6 have been abolished. Bill C-36 has just been pushed through and is almost identical to Bill C-52 and Bill C-6.
Watch Shawn Buckley's Senate Committee Session, Part I and Part II, where he was a witness in opposition to Bill C-6 (now Bill C-36). To view all 10 parts visit YouTube.
Click here for the Red Code alert about Bill C-6, and its call for immediate action by Canadians.
In A Question of Sovereignty, international award winning Writer/Director Kevin P. Miller (GENERATION RX, WE BECOME SILENT) exposes how Canadians are being stripped of their personal sovereignty by government agencies — and how free trade deals and other international agreements imperil Canadian democracy.
Quietly, over a period of many years, unconstitutional legislation encompassed in Bills C-51, C-6, and the current Bill C-36 have placed not only basic civil liberties and freedoms at risk, but Canada's national sovereignty as well. The film shares how entangling alliances with groups like the World Trade Organization, the World Health Organization, Codex Alimentarius, the United States and even multinational corporate interests have become so powerful that they literally threaten to make elected officials in Parliament irrelevant.
A QUESTION OF SOVEREIGNTY discusses why this dramatic shift in the balance of power puts the nation and its people at a vital crossroad early in the 21st century — and why some of the past giants of Canadian politics may hold the answers to Canada's future.
_______________________________________________________
United States of America
US citizens along with the natural products industry came together in a unique effort back in 1994. This mass reaction to the Food and Drug Administration’s attempt to medicalise dietary supplements was simply not acceptable to Americans. Emerging from this people’s revolution, which saw a higher level of citizen protest in Congress than any other issue in US history, was the Dietary Supplement Health and Education Act (DSHEA). This US law not only maintained dietary supplements as a category of food it also prevented their adulteration and allowed for specific structure/function claims linked to disease risk reduction.
Since DSHEA there has been a massive growth of the US natural health product market. It’s becoming increasingly apparent that US regulators, probably in cahoots with their corporate allies in the pharmaceutical industry, are desperate to put the brakes on the dietary supplement industry. There is plenty of evidence of attempts to pass laws that are congruent with the draconian laws of Europe.
There has never been a more important time to defend the US natural products base, particularly given that it acts as the research and development arm for the rest of the world.
Find out all about that’s going on via the ANH-USA website
________________________________________________________
New Zealand
New Zealand citizens have being fighting for the rights to not be forced into a medicalised model, that was imposed in nearby Australia back in 1990, and continues to strangle more and more of the natural health industry.
In the front lines of this work, is ANH-Intl's affiliate, the New Zealand Health Trust.
Although 'Trans-Tasman harmonisation' has recently been beaten in the New Zealand parliament, we know from experience in other parts of the world, such as Europe and Canada, the pressure won't go away. Vigilance is one of the most important requirements for anyone concerned about maintaining their health freedom and freedom of choice in healthcare.
We'll keep you posted on new developments—and those wanting to keep a finger on the NZ pulse—please bookmark the NZ Health Trust website.
On the NZ Health Trust website you will find details with the NZ Ministry of Health requesting submissions on The Development of a Natural Health Products Bill consultation paper.
Read ANH-Intl's consultation response.
________________________________________________________
Why do we need to keep therapeutic natural health products available on the market?
Clinical nutrition, nutritional therapy and plain old good nutritional management have long included using dosages of vitamins and minerals that are substantially higher than those typically found in the average, contemporary diet.
Our bodies' requirement for higher dose supplements
The following set of precepts were formulated and published upon the founding of the British Society for Nutritional Medicine, now the British Society for Ecological Medicine, in June 1984 [1] and modified in the first issue of this journal in 1990, as follows.
- Man's diet, even in industrialized societies, may have only a borderline, or indeed low, content of certain essential nutrients. A 'normal' diet is not necessarily a healthy or optimum one.
- Requirements for essential nutrients vary from individual to individual depending on genetic, physiological, lifestyle and other influences. What is adequate for one person may not be adequate for another.
- Illness is inevitably linked with an abnormal biochemistry and an alteration in the metabolism of nutrients and their by-products.
- Specific nutrients such as vitamins, minerals, essential fatty acids and amino acids, as well as dietary manipulation in general, provide a potent means of influencing body biochemistry and thus disease processes.
- Reproductive processes are nutrient-dependent and sensitive to environmental pollution. Nutritional status of and environmental factors affecting both parents in the preconceptional and periconceptional period, and nutritional status of and environmental factors affecting the mother throughout pregnancy, are primary determinants of pregnancy outcome.
________________________________________________________
The EU is planning to dumb down our food supplements—imminently!
The EU is in the process of developing a regime to control the maximum permitted levels (MPLs) of food supplements EU-wide. The levels are likely to be unnecessarily low—being based on flawed science —an they could prevent you obtaining sufficient nutrients to allow you to manage your health. For example, the level of beta-carotene could be less than that found in just two carrots, and that for selenium, less than the amount found in two brazil nuts.
Can you let this happen?
Find out why you should be concerned and download the ANH position paper on MPLs.
The beta-carotene content of just one large (70 g) raw carrot provides around 7.2 mg of beta-carotene. This amount of beta-carotene might exceed the maximum permitted level (MPL) allowed in supplements across Europe as of late 2010, when MPLs find their way into EU law.
How the European institutions are responding?
Find out how the Irish Association for Health Stores petition is playing a critical role in bringing sense, good science and democracy to the process. In January 2009, the European Parliament's Petitions Committee voted to keep the petition open and referred it to the key parliamentary group dealing with matters of health and consumer protection.
Markos Kyprianou, the (unelected) European Commissioner responsible for health and consumer protection, made clear in January 2007 that the European Commission does not wish to allow sale of therapeutically active food supplements.
So, while many countries in Europe have regulated maximum levels of vitamins and minerals via multiples of the Recommended Daily Allowance (RDA), the European Commission and the European Food Safety Authority (EFSA) is in the late stages of planning the methods it will use to develop EU-wide, harmonised, maximum permitted levels for food supplements and fortified foods. This process will be instigated legally through an implementing measure of the Food Supplements Directive.
A harmonised European market for maximum (and minimum) dosages will no doubt be of considerable benefit to large corporations, especially the pharmaceutical companies that dominate the low-dose end of the supplement market, as they will no longer need to reformulate products for specific EU markets.
Low doses EU-wide are also regarded by many regulators as a useful end-point for meeting the Food Supplements Directive’s requirements for a “high level of consumer protection”. Nutritional therapists may, of course, take a different view, since they are much more likely to recognise the two-tailed nature of risks associated with nutrients. At very low levels of intake there are risks of inadequacy (that go well beyond simply the risk of developing deficiency diseases, but of course significantly impact the risk of chronic and even infectious diseases). At high levels of intake of some nutrient forms, there are, of course, potential risks associated with excessive intake. This may be particularly true of certain fat soluble vitamins, especially synthetic, isolated forms, and also some minerals, that have a somewhat narrow beneficial and therapeutic dose range (e.g. selenium, vanadium). Complicating matters even further, practitioners are also much more cognisant of the differences between short and long-term exposure. Short-term, high dose therapy with certain nutrients may in fact be highly beneficial (e.g., B vitamins), while the risks associated with long-term exposure, which are generally mild, transient and fully reversible, unlike many side effects associated with pharmaceutical drugs, are well recognised.
So how is the EU looking to take into account these differing requirements? An obvious approach, which has never been seriously contemplated by the EU authorities, is to develop a bespoke regime for practitioners—a third category, that exists between food and medicines. Whether the absence of a ‘third category’ has been the result of pressure from the pharmaceutical industry, inadequate pressure from practitioners and their associations, a requirement for simplification of the legal regimes by the regulators, or a combination of these elements, is anybody’s guess. But, all the evidence thus far suggests that a system is in development that utilises the formulaic, one-size-its-all approach already laid out in Article 5 of the Food Supplements Directive, which is likely to result in extremely low daily levels of many vitamins and minerals.
The Federal Institute of Risk Assessment in Germany (the Bundesinstitut für Risikobewertung, or BfR) has already employed their interpretation of Article 5 to determine maximum permitted levels (MPLs) and the results emphasise the concerns of many objective scientists.
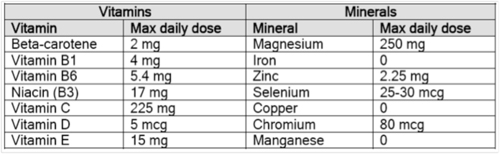
Some proposed MPLs (daily doses) for food supplements, as determined by the BfR, are listed below:
Putting the BfR values into perspective....
- A 200 g sirloin steak gives you around 7.2 mg of zinc, over 3 times the BfR maximum level of zinc in food supplements
- A single large raw carrot (70 g) typically contains 7.2 mg of beta-carotene (601 mcg Retinol Equivalents), around 3.6 times the BfR maximum level of beta-carotene in food supplements
- One single (5 g) brazil nut typically contains 96 mcg of selenium, over 3 times the BfR maximum level for selenium in food supplements
- One cup of raw french beans (180 g) gives you about 346 mg magnesium, 3.5 mg zinc and 734 mcg folate—these are all above or well above the BfR maximum levels for these three nutrients!
[the above determinations have been made using the USDA National Nutrient Database]
Fortunately, there have been many objections to the BfR approach to risk assessment and management for the determination of MPLs, but other options that are under consideration still, in the main, reveal MPLs, and in several important cases even Safe Upper Levels (SULs) for most vitamins and minerals that are well beneath the therapeutic range. A dramatic reminder of how cock-eyed these risk-based assessments are is given by comparing MPLs with amounts found in our food. For example, and astonishingly, the beta-carotene in two carrots or the selenium in one Brazil nut will typically exceed the MPLs for beta-carotene and selenium respectively.
Maximum Permitted Levels, or even SULs, insinuate that higher levels may expose consumers to risk, so most people assume that therapeutic ranges would typically be risky. Several decades of clinical nutritional practice demonstrates that the therapeutic range, just like the beta-carotene in ten carrots, or a handful of brazil nuts, poses no risk and is, as the term ‘therapeutic’ would imply, beneficial to health.
The ANH-Intl has submitted detailed submissions to European Commission, EFSA and the UK Food Standards Agency, drawing attention to some of the limitations of the proposed methodologies and suggesting alternatives, that are based on rational science. It is of paramount importance that, in determining MPLs, due account is given to other factors and processes. These include:
- the speciation of the nutrient (different nutrient forms of the same nutrient [group] often follow distinct pathways in the body, resulting in differing bioavailability, metabolism and toxicology) ;
- medical records and other medical or scientific evidence which demonstrates the safety of therapeutic and beneficial dosages of nutrients;
- evidence of safety of high intakes of nutrients ingested in foods by specific population groups;
- determinations must take into account all available and relevant evidence on both risks and benefits and must not ignore relevant studies and case reports;
- Where SULs cannot be established through lack of data, it is not possible to determine MPLs. Guidance Levels, as determined by the Expert Group on Vitamins and Minerals (EVM) should not be used as surrogates for SULs.
- MPLs should be waived in situations where there are inadequate data to produce scientifically meaningful SULs or where there is no evidence of toxicity at even very high dosages (e.g., thiamine, riboflavin, vitamin B12, biotin, etc.). As such, regulation needs to be proportionate and should only be applied where genuine risks to the general public can be determined.
________________________________________________________
Making different health choices can change the world.....
Just imagine what would happen if we all just followed the 10 simple behaviours listed below.
- Take responsibility for your own health, foster awareness and lead by example
- Eat a diet that's varied, balanced and made up largely of whole foods—choose not to buy processed or GM foods
- Buy/cultivate local/regional whole, organic/biodynamic/sustainable foods, including heirloom varieties—avoid relying on supermarkets as your primary source of food if possible or at least choose wisely
- Enjoy a healthy lifestyle—exercise moderately, breathe fresh air, drink clean water, manage stress effectively, get enough quality sleep, minimise your exposure to toxins and harmful radiation sources as much as possible
- Micronutrients—prefer fully natural products and ingredients
- Support ethically sound, enviromentally friendly companies and products
- Where possible support the self-healing capacities of your body and try to choose a healthcare practitioner/doctor who is qualified in and respects the fields of medicine you believe are most appropriate to your needs. Or you can educate your doctor—do your own research and show him/her the options that are out there. That's Health Choice!
- Communicate the challenges and ways to overcome them to ensure that our fundamental right to natural health is not eroded. Awareness and education offer empowerment to make positive change
- Act local, think global! Focus on the ‘next generation’ and work with kids—help them to understand and get involved with nature, natural food and wholefood preparation
- Support pro-health freedom, pro-organic, anti-GM organisations—these organisations are on the front lines on your behalf.